Dec 1, 2020 By: yunews
 Dr. Ran Drori
Dr. Ran Drori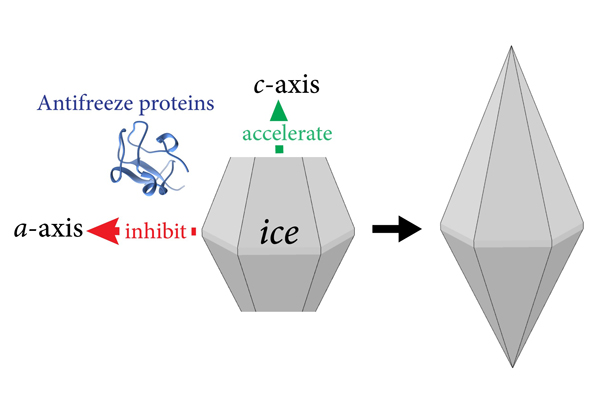 The team was perplexed by the different effects on the ice crystal growth. If AFPs are meant to prevent ice growth within organisms, then why would some accelerate the growth velocity?
The answer to this question is found in the shape of the ice crystal exposed to AFPs.
These proteins cannot bind and inhibit all the surfaces of the crystal; instead, they inhibit growth in some crystallographic direction while promoting growth of other directions. In this way, a bipyramidal-shaped crystal is obtained, and the surfaces that the AFPs cannot inhibit are the sharp tips of the bipyramidal crystal. “So, growth acceleration helps to achieve the desired bipyramidal crystal faster and at higher temperatures, which in turn stops further ice growth and prevents freezing injuries to organisms,” Dr. Drori explained.
NOTE: The authors dedicate this paper to Dr. Lea Blau a"h, Professor Emerita of Chemistry and Biochemistry at Stern College for Women, Yeshiva University, who passed away in May 2020. She was the heart and soul of the department that she led for 36 years, retiring in 2015. She was a passionate teacher and researcher and mentored countless undergraduate students as well as junior faculty and was a source of strength, dedication, and kindness at the college.
The team was perplexed by the different effects on the ice crystal growth. If AFPs are meant to prevent ice growth within organisms, then why would some accelerate the growth velocity?
The answer to this question is found in the shape of the ice crystal exposed to AFPs.
These proteins cannot bind and inhibit all the surfaces of the crystal; instead, they inhibit growth in some crystallographic direction while promoting growth of other directions. In this way, a bipyramidal-shaped crystal is obtained, and the surfaces that the AFPs cannot inhibit are the sharp tips of the bipyramidal crystal. “So, growth acceleration helps to achieve the desired bipyramidal crystal faster and at higher temperatures, which in turn stops further ice growth and prevents freezing injuries to organisms,” Dr. Drori explained.
NOTE: The authors dedicate this paper to Dr. Lea Blau a"h, Professor Emerita of Chemistry and Biochemistry at Stern College for Women, Yeshiva University, who passed away in May 2020. She was the heart and soul of the department that she led for 36 years, retiring in 2015. She was a passionate teacher and researcher and mentored countless undergraduate students as well as junior faculty and was a source of strength, dedication, and kindness at the college.